https://doi.org/10.1140/epje/s10189-023-00380-w
Regular Article - Flowing Matter
Chilling alcohol on the computer: isothermal compressibility and the formation of hydrogen-bond clusters in liquid propan-1-ol
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128, Mainz, Germany
Received:
22
July
2023
Accepted:
15
November
2023
Published online:
29
November
2023
Molecular dynamics simulations have been performed to compute the isothermal compressibility 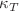 of liquid propan-1-ol in the temperature range
of liquid propan-1-ol in the temperature range 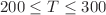 K. A change in behaviour, from normal (high T) to anomalous (low T), has been identified for
K. A change in behaviour, from normal (high T) to anomalous (low T), has been identified for 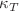 at
at 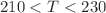 K. The average number of hydrogen bonds (H–bond) per molecule turns to saturation in the same temperature interval, suggesting the formation of a relatively rigid network. Indeed, simulation results show a strong tendency to form H–bond clusters with distinct boundaries, with the average largest size and width of the size distribution growing upon decreasing temperature, in agreement with previous theoretical and experimental studies. These results also emphasise a connection between the behaviour of
K. The average number of hydrogen bonds (H–bond) per molecule turns to saturation in the same temperature interval, suggesting the formation of a relatively rigid network. Indeed, simulation results show a strong tendency to form H–bond clusters with distinct boundaries, with the average largest size and width of the size distribution growing upon decreasing temperature, in agreement with previous theoretical and experimental studies. These results also emphasise a connection between the behaviour of 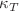 and the formation of nanometric structures.
and the formation of nanometric structures.
Luis A. Baptista and Mauricio Sevilla have contributed equally to this work.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1140/epje/s10189-023-00380-w.
© The Author(s) 2023
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.





