https://doi.org/10.1140/epje/s10189-023-00360-0
Regular Article - Living Systems
Pincus blob elasticity in an intrinsically disordered protein
1
Materials Department, University of California, Santa Barbara, USA
2
The Raymond and Beverly Sackler School of Physics and Astronomy and The Center for Nanoscience and Nanotechnology, Tel Aviv University, Tel Aviv, Israel
3
The Center of Physics and Chemistry of Living Systems, Tel Aviv University, Tel Aviv, Israel
4
Biomolecular Science and Engineering Program, University of California, Santa Barbara, USA
5
Physics Department, University of California, Santa Barbara, USA
Received:
15
July
2023
Accepted:
29
September
2023
Published online:
17
October
2023
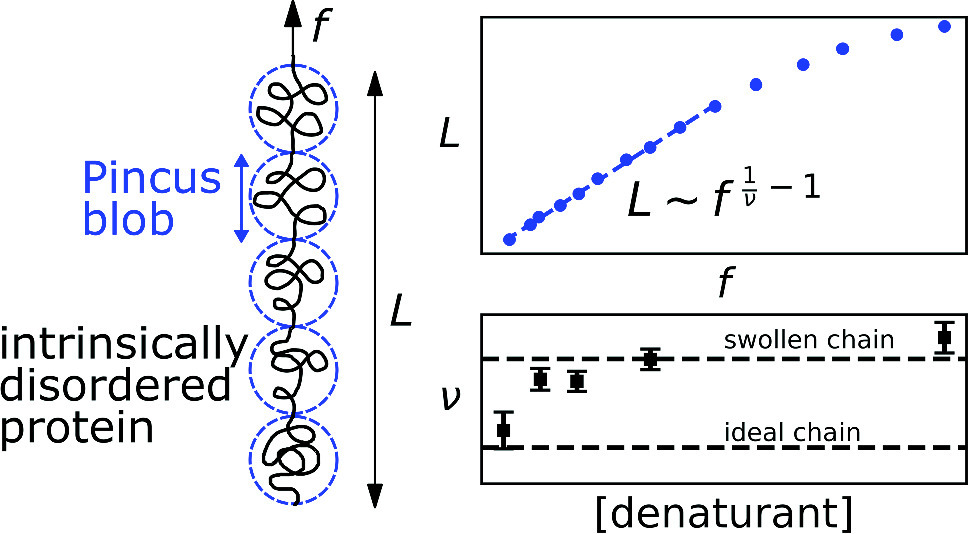
Understanding the dynamic structure of intrinsically disordered proteins (IDPs) is important to deciphering their biological functions. Here, we exploit precision entropic elasticity measurements to infer the conformational behavior of a model IDP construct formed from the disordered tail of the neurofilament low molecular weight protein. The IDP construct notably displays a low-force power-law elastic regime, consistent with the Pincus blob model, which allows direct extraction of the Flory exponent,  , from the force–extension relationship. We find
, from the force–extension relationship. We find  increases with added denaturant, transitioning from a nearly ideal chain to a swollen chain in a manner quantitatively consistent with measurements of IDP dimensions from other experimental techniques. We suggest that measurements of entropic elasticity could be broadly useful in the study of IDP structure.
increases with added denaturant, transitioning from a nearly ideal chain to a swollen chain in a manner quantitatively consistent with measurements of IDP dimensions from other experimental techniques. We suggest that measurements of entropic elasticity could be broadly useful in the study of IDP structure.
The original online version of this article was revised: This article was updated to include a co-author: Ian L. Morgan, who is omitted in the initial version due to an oversight. The original article has been corrected.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1140/epje/s10189-023-00360-0.
A correction to this article is available online at https://doi.org/10.1140/epje/s10189-023-00382-8.
Copyright comment corrected publication 2023
Copyright comment corrected publication 2023
Copyright comment Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
© The Author(s), under exclusive licence to EDP Sciences, SIF and Springer-Verlag GmbH Germany, part of Springer Nature 2023. corrected publication 2023. corrected publication 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.