https://doi.org/10.1140/epje/s10189-023-00301-x
Regular Article - Living Systems
Uncovering diffusive states of the yeast membrane protein, Pma1, and how labeling method can change diffusive behavior
1
Integrated Graduate Program in Physical and Engineering Biology, Yale University, 06511, New Haven, CT, USA
2
Department of Applied Physics, Yale University, 06511, New Haven, CT, USA
3
Department of Physics, Yale University, 06511, New Haven, CT, USA
4
Department of Cell Biology, Yale University, 06511, New Haven, CT, USA
5
Department of Biomedical Engineering, Yale University, 06511, New Haven, CT, USA
6
Institute of Quantitative Biology, Biochemistry and Biotechnology, Center for Synthetic and Systems Biology, School of Biological Sciences, University of Edinburgh, 06511, Edinburgh, UK
Received:
30
August
2022
Accepted:
15
May
2023
Published online:
9
June
2023
We present and analyze video-microscopy-based single-particle-tracking measurements of the budding yeast (Saccharomyces cerevisiae) membrane protein, Pma1, fluorescently labeled either by direct fusion to the switchable fluorescent protein, mEos3.2, or by a novel, light-touch, labeling scheme, in which a 5 amino acid tag is directly fused to the C-terminus of Pma1, which then binds mEos3.2. The track diffusivity distributions of these two populations of single-particle tracks differ significantly, demonstrating that labeling method can be an important determinant of diffusive behavior. We also applied perturbation expectation maximization (pEMv2) (Koo and Mochrie in Phys Rev E 94(5):052412, 2016), which sorts trajectories into the statistically optimum number of diffusive states. For both TRAP-labeled Pma1 and Pma1-mEos3.2, pEMv2 sorts the tracks into two diffusive states: an essentially immobile state and a more mobile state. However, the mobile fraction of Pma1-mEos3.2 tracks is much smaller (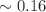 ) than the mobile fraction of TRAP-labeled Pma1 tracks (
) than the mobile fraction of TRAP-labeled Pma1 tracks ( ). In addition, the diffusivity of Pma1-mEos3.2’s mobile state is several times smaller than the diffusivity of TRAP-labeled Pma1’s mobile state. Thus, the two different labeling methods give rise to very different overall diffusive behaviors. To critically assess pEMv2’s performance, we compare the diffusivity and covariance distributions of the experimental pEMv2-sorted populations to corresponding theoretical distributions, assuming that Pma1 displacements realize a Gaussian random process. The experiment–theory comparisons for both the TRAP-labeled Pma1 and Pma1-mEos3.2 reveal good agreement, bolstering the pEMv2 approach.
). In addition, the diffusivity of Pma1-mEos3.2’s mobile state is several times smaller than the diffusivity of TRAP-labeled Pma1’s mobile state. Thus, the two different labeling methods give rise to very different overall diffusive behaviors. To critically assess pEMv2’s performance, we compare the diffusivity and covariance distributions of the experimental pEMv2-sorted populations to corresponding theoretical distributions, assuming that Pma1 displacements realize a Gaussian random process. The experiment–theory comparisons for both the TRAP-labeled Pma1 and Pma1-mEos3.2 reveal good agreement, bolstering the pEMv2 approach.
Mary Lou P. Bailey and Susan E. Pratt have contributed equally to this work.
Copyright comment Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
© The Author(s), under exclusive licence to EDP Sciences, SIF and Springer-Verlag GmbH Germany, part of Springer Nature 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.





