https://doi.org/10.1140/epje/s10189-022-00250-x
Regular Article - Living Systems
Key interaction patterns in proteins revealed by cluster expansion of the partition function
1
Department of Physics, Università degli Studi di Milano, Via Celoria 16, 20133, Milan, Italy
2
Department of Physics and Astronomy “G. Galilei”, Università degli Studi di Padova and INFN, Via Marzolo 8, 35121, Padova, Italy
3
Department of Physics and Center for Complexity and Biosystems, Università degli Studi di Milano and INFN, Via Celoria 16, 20133, Milan, Italy
Received:
29
September
2022
Accepted:
19
November
2022
Published online:
29
November
2022
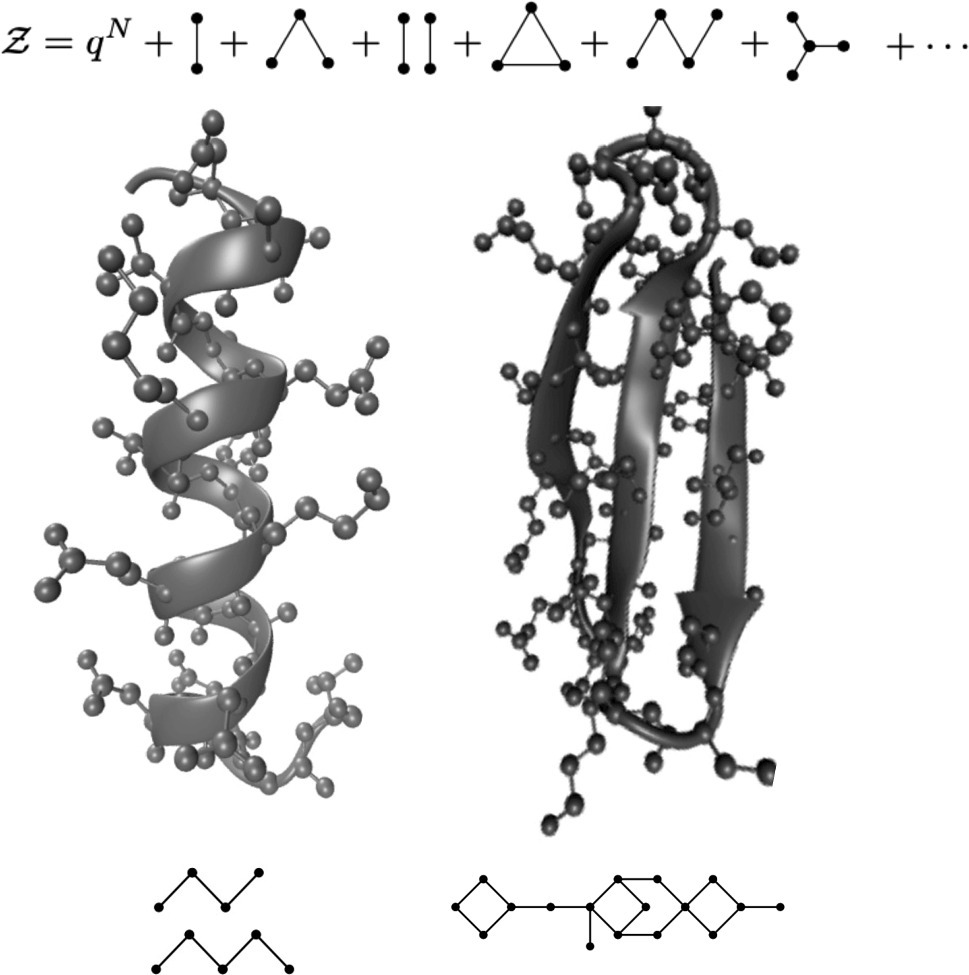
The native conformation of structured proteins is stabilized by a complex network of interactions. We analyzed the elementary patterns that constitute such network and ranked them according to their importance in shaping protein sequence design. To achieve this goal, we employed a cluster expansion of the partition function in the space of sequences and evaluated numerically the statistical importance of each cluster. An important feature of this procedure is that it is applied to a dense finite system. We found that patterns that contribute most to the partition function are cycles with even numbers of nodes, while cliques are typically detrimental. Each cluster also gives a contribute to the sequence entropy, which is a measure of the evolutionary designability of a fold. We compared the entropies associated with different interaction patterns to their abundances in the native structures of real proteins.
Copyright comment Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
© The Author(s), under exclusive licence to EDP Sciences, SIF and Springer-Verlag GmbH Germany, part of Springer Nature 2022. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.