https://doi.org/10.1140/epje/i2016-16086-5
Regular Article
Thermophoresis of cyclic oligosaccharides in polar solvents
1
School of Science, Tokai University, 259-1292, Kanagawa, Japan
2
ICS-3 Soft Condensed Matter, Forschungszentrum Juelich GmbH, D-52428, Juelich, Germany
3
Chemistry Department - Physical Chemistry, University Cologne, D-50939, Cologne, Germany
4
Micro/Nano Technology Center, Tokai University, 259-1292, Kanagawa, Japan
* e-mail: d.niether@fz-juelich.de
** e-mail: rkita@keyaki.cc.u-tokai.ac.jp
Received:
21
July
2016
Accepted:
5
September
2016
Published online:
27
September
2016
Cyclodextrins are cyclic oligosaccharides which are interesting as drug delivery systems, because they can be used as containers for pharmaceutical substances. We studied the Ludwig-Soret effect of 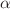 -,
-, 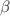 -,
-,  - and methyl-
- and methyl-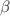 -cyclodextrin in water and formamide by infrared thermal diffusion forced Rayleigh scattering (IR-TDFRS). In water the Soret coefficient, S
T, of
-cyclodextrin in water and formamide by infrared thermal diffusion forced Rayleigh scattering (IR-TDFRS). In water the Soret coefficient, S
T, of 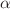 -,
-, 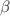 - and
- and  -cyclodextrin increases with increasing temperature and shows a sign change from negative to positive around T = 35 °
C, while S
T of methyl-
-cyclodextrin increases with increasing temperature and shows a sign change from negative to positive around T = 35 °
C, while S
T of methyl-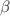 -cyclodextrin is positive in the entire investigated temperature. In formamide S
T-values of all cyclodextrins coincide and show a slight decrease with temperature. We discuss the obtained results and relate the S
T-values to the different hydrogen bonding capabilities of the cyclodextrins and the used solvents. It turns out that the change of S
T with temperature correlates with the partition coefficient, logP, which indicates that more hydrophilic substances show a more pronounced temperature sensitivity of S
T. Additionally we obtained a surprising result measuring the refractive index contrast factor with temperature,
-cyclodextrin is positive in the entire investigated temperature. In formamide S
T-values of all cyclodextrins coincide and show a slight decrease with temperature. We discuss the obtained results and relate the S
T-values to the different hydrogen bonding capabilities of the cyclodextrins and the used solvents. It turns out that the change of S
T with temperature correlates with the partition coefficient, logP, which indicates that more hydrophilic substances show a more pronounced temperature sensitivity of S
T. Additionally we obtained a surprising result measuring the refractive index contrast factor with temperature, 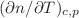 of cyclodextrins in formamide, which might be explained by a complex formation between cyclodextrins and formamide.
of cyclodextrins in formamide, which might be explained by a complex formation between cyclodextrins and formamide.
Key words: Topical Issue: Non-isothermal transport in complex fluids
© EDP Sciences, SIF, Springer-Verlag Berlin Heidelberg, 2016





