Eur. Phys. J. E 7, 233-240 (2002)
DOI: 10.1140/epje/i200101073
Growth kinetics of self-assembled monolayers
of thiophene and terthiophene on
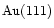 : An infrared spectroscopic
study
: An infrared spectroscopic
study
T. Matsuura1 and Y. Shimoyama2
1 Department of Applied Physics, Graduate School of Engineering, Hokkaido University, Sapporo 060-8628, Japan
2 Department of Physics, Hokkaido University of Education, Hakodate 040-8567, Japan
yuhei@cc.hokkyodai.ac.jp
(Received 23 May 2001 and Received in final form 11 February 2002)
Abstract
The growth kinetics of self-assembled monolayers (SAMs) of thiophene
compounds on Au(111) surfaces was revealed by Fourier-transform infrared
reflection absorption spectroscopy (FT-IR-RAS).
Thiophene and terthiophene form well-ordered SAMs on Au(111) surfaces
by immersing gold substrates into their ethanol solutions for ca. 15 h.
Gibbs free energies for the adsorption processes of thiophene and
terthiophene were found to be identical.
However, the growth and molecular orientation of SAMs are different
between two thiophene compounds.
Terthiophene in SAMs orients parallel to the surface.
The SAM growth of terthiophene obeys a time-dependent Langmuir scheme.
On the other hand, the thiophene SAM undergoes a two-step growth
process with unique molecular orientations.
In the primary phase, thiophene assumes a parallel orientation on the Au(111) surface.
In the second phase, thiophene is oriented close to the normal of the surface.
The different growth process between thiophene and terthiophene is
attributable to the topology of sulfur positions in the molecules.
68.55.-a - Thin film structure and morphology.
81.10.Aj - Theory and models of crystal growth; physics of crystal growth, crystal morphology and orientation.
78.30.-j - Infrared and Raman spectra.
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag 2002







