https://doi.org/10.1140/epje/i2018-11720-x
Regular Article
Structural aspects of human lactoferrin in the iron-binding process studied by molecular dynamics and small-angle neutron scattering
1
Institute of Chemistry (ICh), Academiei 3, MD-2028, Chisinau, Republic of Moldova
2
Jülich Centre for Neutron Science (JCNS) at Heinz Maier-Leibnitz Zentrum (MLZ), Forschungszentrum Jülich GmbH, Lichtenbergstraße 1, 85748, Garching, Germany
3
Neutron materials characterization (NØYTRON), Institute for Energy Technology (IFE), Instituttveien 18, P.O. Box 40, 2027, Kjeller, Norway
4
Horia Hulubei National Institute for R&D in Physics and Nuclear Engineering (IFIN-HH), Reactorului 30, P.O. Box MG-6, Bucharest - Magurele, Romania
* e-mail: lilia.anghel@chem.asm.md
** e-mail: raul.erhan@ife.no
Received:
12
April
2018
Accepted:
28
August
2018
Published online:
20
September
2018
Lactoferrin is a non-heme protein known for its ability to bind tightly Fe(III) ions in various physiological environments. Due to this feature lactoferrin plays an important role in the processes of iron regulation at the cellular level preventing the body from damages produced by high levels of free iron ions. The X-ray crystal structure of human lactoferrin shows that the iron-binding process leads to conformational changes within the protein structure. The present study was addressed to conformation stability of human lactoferrin in solution. Using molecular dynamics simulations, it was shown that Arg121 is the key amino acid in the stabilization of the Fe(III) ion in the N-lobe of human lactoferrin. The small-angle neutron scattering method allowed us to detect the structural differences between the open and closed conformation of human lactoferrin in solution. Our results indicate that the radius of gyration of apolactoferrin appears to be smaller than that of the hololactoferrin, 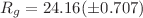 Å and
Å and 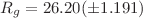 Å, respectively. The low-resolution three-dimensional models computed for both forms of human lactoferrin in solution also show visible differences, both having a more compact conformation compared to the high-resolution structure.
Å, respectively. The low-resolution three-dimensional models computed for both forms of human lactoferrin in solution also show visible differences, both having a more compact conformation compared to the high-resolution structure.
Key words: Soft Matter: Self-organisation and Supramolecular Assemblies
© The Author(s), 2018





